Biomineralization, the complex process leading to the formation of organized mineral crystals, persists throughout life and is fundamental in bone formation, repair, and remodeling.
In human bone mesenchymal stem cells (bMSC), osteoblasts’ precursors, we have shown that biomineralization starts within the cell with the nucleation of Zn–hydroxyapatite, and then rapidly evolves toward hydroxyapatite crystals, with composition and structure similar to the one present in human bone.
To this aim, Crystallography was jointly used with the Small and Wide Angle X-ray Scattering scanning microscope, available at the X-ray Micro-Imaging Laboratory (XMI-L@b) as in vitro diagnostic tools to study and classify the crystal and chemical nature of the Zn-hydroxyapatite calcifications. bMSC, rare pluripotent cells that activate the genetic program leading to osteoblastogenesis in response to specific chemical or physical stimuli, can migrate into sites of injury, self-renew, and differentiate as well as release trophic and growth factors.
For these reasons bMSC are prime candidates for use in regenerative medicine. Moreover, bMSC are critical for the osteointegration of orthopedic implants .

Two-dimensional X-rayfluorescence maps (pixel size 70 nm, expressed in areal mass (g/cm2)) showing the evolution between day 4 andday 10 of bMSC osteogenic differentiation. (A, E) Red: elemental maps of Ca. (B, F) Green: elemental maps of P. (C, G) Blue: elemental maps ofZn. (D, H) Composite elemental distribution of Ca, P, and Zn to better understand the correspondence of elements accumulation [Procopio et al., ACS Central Science 20195 (8), 1449-1460, DOI:10.1021/acscentsci.9b00509]
With senescence, a numerical decline and a functional impairment of bMSC has been reported, resulting in bone diseases that are common in aged population and are among the main causes of disability and morbidity. The mechanisms of age-related changes in bMSC have not been fully characterized. It is known that senescent human bMSC are irreversibly growth arrested, genetically reprogrammed, morphologically altered, show increased activity of senescence-associated βgalactosidase, and lose osteogenic potential. Interestingly, in a murine model, senolytics improved osteogenic capacity of aged BMSC both in vitro and in vivo. Cellular senescence is a critical issue since it is a ubiquitous feature of cells derived from regenerative somatic tissue and a primary causal mechanism of ageing and age-related disease. Indeed, senescence is not a “tissue culture artefact”, and senescent cells are present in many different tissues in vivo.
Project Main Goal
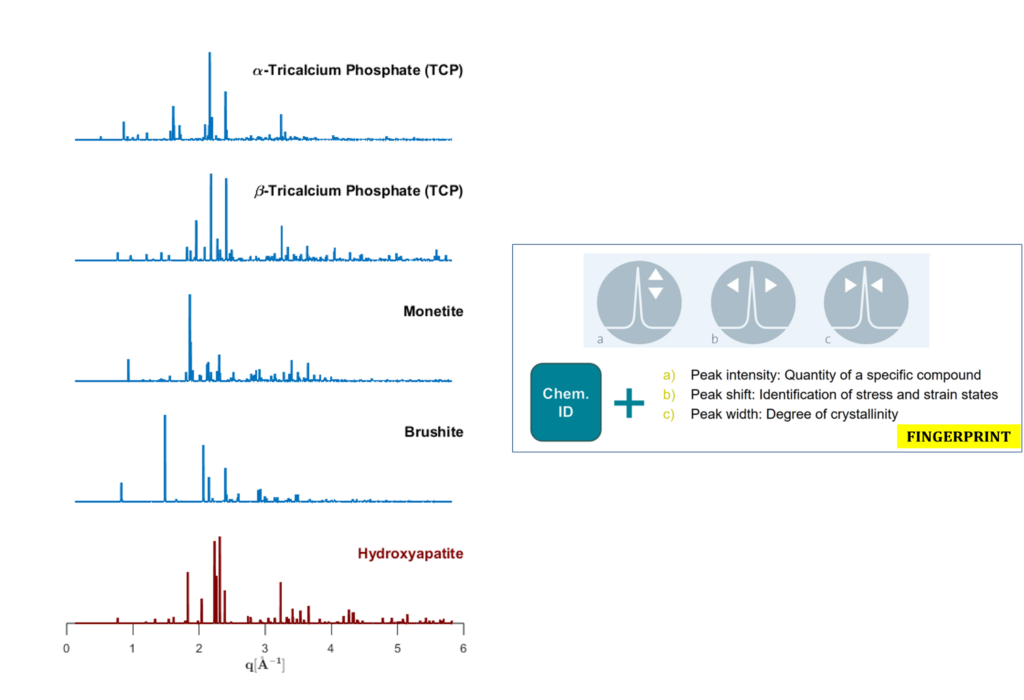
The main goal of the Project is to study the in vitro behavior of young and senescent bMSC at the supra- and sub-molecular level to assign uniquely the correct identity of the inorganic calcium phosphate aggregates, deposited by the young, middle-aged and senescent cells.
Indeed, all calcium-phosphate salts present similar composition, but have a different arrangement of the atoms in the crystal lattice. The specific role of some crucial metallic components in the hydroxyapatite formation – Mg, Zn and Cu – in young and senescent bMSC will be explored in order to interpret thoroughly the results obtained by microimaging.
